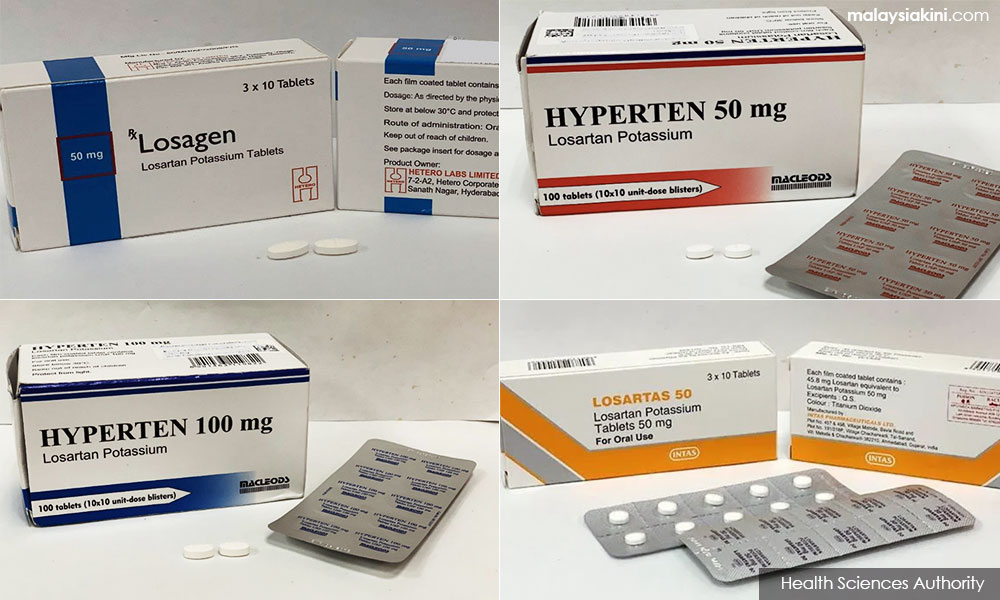
Patients who still have stock of high blood pressure medicines containing the active ingredient losartan are advised not to panic and to return them to suppliers, Deputy Health Minister Dr Lee Boon Chye said today.
He said the suppliers have been prohibited from selling the medicines effective March 7, after the Health Ministry suspended the registration of seven drug products containing the substance.
"When consumed over a long period, the medication can be a carcinogen, but it is of no health threat when used for a short term.
"Those who have been on it need not panic, just return them to the suppliers to have them replaced with other alternatives.
"By right the suppliers should no longer have stock of the products, as we have recalled all the old stocks,“ he told reporters after launching the state-level World Kidney Day celebrations at Meru today.
The seven products which have been suspended are Losagen 50 (Losartan potassium tablets 50mg), Losagen 100 (Losartan potassium tablets 100mg), Losartas 50mg (Losartan potassium tablets 50mg), and Losartas 100mg tablets (Losartan potassium tablets 100mg).
Also in the list are Tozaar Plus LS 50/12.5mg tablet (Losartan potassium/ hydrochlorothiazide); Tozaar 50mg tablets (Losartan potassium tablets 50mg) and Tozaar 100mg tablets (Losartan potassium tablets 100mg).
Yesterday, Health director-general Dr Noor Hisham Abdullah in a statement said the products with the active substance losartan from Hetero Labs Limited, India were suspended in Malaysia since March 7, 2019.
Elaborating, Lee said the ministry was constantly monitoring and collaborating with foreign agencies on medicines marketed in the country.
In another development, Lee, the Gopeng MP, said the ministry has identified over three million people aged 50 and above who are eligible for the B40 Health Protection Scheme (PekaB40) to be launched next month.
"For the initial stage, we have allocated RM100 million for 800,000 participants. If the response is good we will increase the allocation," he said.
- Bernama